The harrowing experience of baby Piper, hospitalized with botulism after consuming ByHeart formula, underscores a growing crisis for the infant formula industry. Piper is one of at least 31 infants across the US suspected or confirmed to have been poisoned by botulism after using ByHeart products, a startup that had promised "clean" and "natural" alternatives.
Hanna Everett recounted that her daughter, Piper, began using ByHeart formula at about two months old. Early this month, Piper developed concerning symptoms, including constipation, excessive drooling, and a drooping left eye. Everett's concerns escalated when a friend sent her a link to the ByHeart formula recall notice. "Sure enough, the can of formula the baby had just finished that day was from the affected lot number", the Richmond, Kentucky resident told NBC News on 22/11.
Piper was admitted to the hospital on 9/11 and diagnosed with botulism. Everett described her shock and helplessness as medical staff worked to stabilize her infant. "They are holding your child, who is not even four months old. She is screaming, and you can't do anything", Everett said, her voice choked with emotion. Piper received botulism antitoxin intravenously, a specialized medication that had to be airlifted to the hospital. Though Piper has since been discharged, Everett remains tormented by guilt. "It feels like I let her down, even though that's not the truth", she stated.
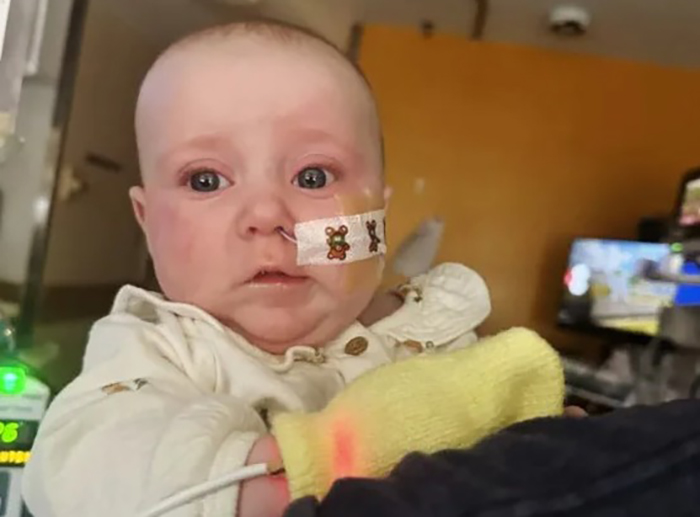 |
Baby Piper receiving treatment in the hospital. *Photo: Hanna Everett* |
The Everett family, Hanna and Michael, filed a lawsuit against ByHeart last week, seeking compensation for medical expenses and emotional and physical damages. Hanna Everett stated that she contacted ByHeart about the recall while Piper was hospitalized, and the company offered to send the family more cans of formula.
Piper's ordeal is not an isolated incident. In Washington, Wescott’s two-month-old daughter was rushed to the emergency room with prolonged constipation and lethargy, classic signs of the toxin paralyzing intestines and muscles. In California, Anthony Barbera and Thalia Flores’s son became so weak he could not cry or breastfeed. In Arizona, Stephen Dexter’s daughter required emergency helicopter transport after being unable to eat.
Botulism is a rare but dangerous condition caused by toxins from the bacterium Clostridium botulinum. According to the Mayo Clinic, this toxin directly attacks the nervous system, preventing the release of acetylcholine, a neurotransmitter that controls muscles. This leads to muscle paralysis, breathing difficulties in infants, and, without prompt intervention with specific antitoxin medication, can be fatal.
The issue came to light when ByHeart, in a statement on 19/11, acknowledged that laboratory tests detected Clostridium botulinum spores in their formula samples. However, the company's initial response was slow and defensive. When the FDA first warned of a potential link between the product and the outbreak, ByHeart only issued a limited recall of two product lots. A day later, the company even posted an announcement claiming insufficient conclusive evidence, arguing that the positive sample was from an opened can and "could have been contaminated from an external source." On its website, ByHeart stated it has not yet identified the cause of the contamination but has shared its test results with the FDA. "We immediately informed the FDA of these findings and are working to investigate the facts, conducting continuous testing to determine the source of contamination", the company stated.
Professor Darin Detwiler, a food policy expert at Northeastern University, noted that ByHeart failed in risk management. He believes the company should have identified the issue and immediately disclosed it transparently instead of waiting for authorities to intervene.
 |
ByHeart formula. *Photo: AP* |
This incident deals a significant blow to the formula industry, where safety must be absolute. Attorney Bill Marler, representing the plaintiff families, emphasized that if any product cannot afford errors, it is infant food. More concerning, this is not an isolated incident for ByHeart. In 12/2022, the company had to recall products due to suspected Cronobacter sakazakii contamination. In 2023, the FDA sent a warning letter about "serious violations" at ByHeart's manufacturing facility in Pennsylvania, where the company also tried to blame the testing process when bacteria were detected.
Although ByHeart asserts that the Pennsylvania facility is not linked to this contaminated batch and commits to investigating the root cause, consumer trust has fractured. For mothers like Wescott, the incident is not just about their children's physical suffering but also a collapse of faith in the food safety system, leaving them in a dilemma: unable to breastfeed sufficiently and no longer daring to trust any formula on the shelves.
Binh Minh (According to NBC News, Mayo Clinic)