The DAV announced the recall on 23/7, citing discrepancies between the products' formulas and their registered documentation. The products' labels also misrepresented their true nature and function. As a result, the DAV has suspended the product registration process for Van Minh Company, the distributor responsible for bringing these products to the Vietnamese market, for six months.
Product registration numbers are issued by the Vietnamese government when a company registers a new product. These numbers confirm that the product has been declared by the responsible organization or individual before being circulated in the market. These registration numbers are valid for five years from the date of issuance. Authorities will revoke the number if the organization or individual requests it, or if the product violates regulations.
The recalled products include: Bel-Energen Daydream Cleansing Foam; Sun Protection/SPF 50+/Very High; Line A Cream; Bel-Energen PhytoSensation Elixir; Intensa Skin Renewal Oleo Serum 10%; Line A Clarity Concentrate; Samtea perfect recovery Body cream; and Sun protection face elixir SPF 30.
These products are sold at relatively high prices in Vietnam. For example, the Samtea perfect recovery Body cream retails for nearly 2.7 million VND, and the Intensa Skin Renewal Oleo Serum costs over 2.8 million VND.
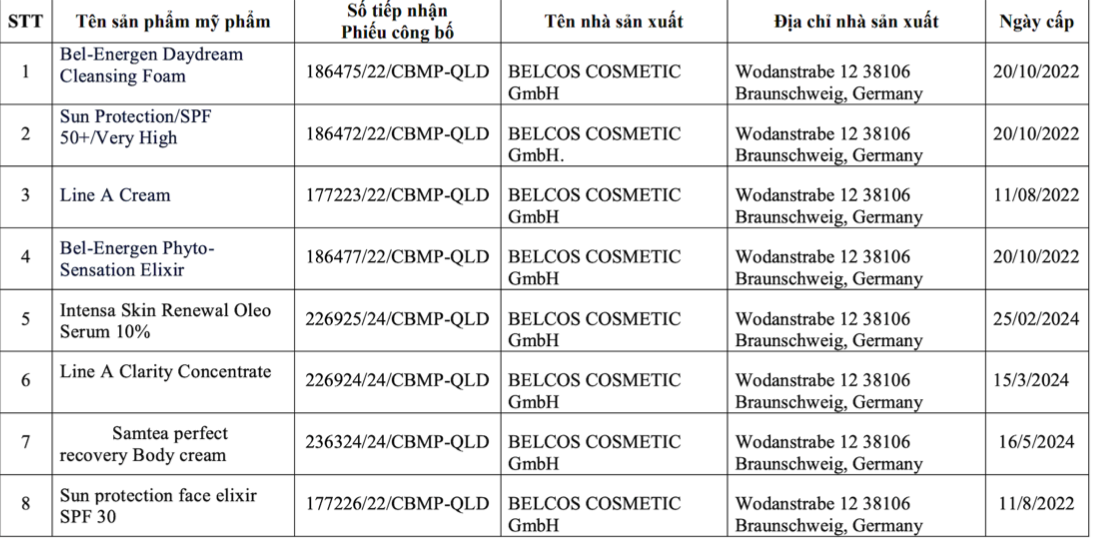 |
List of the 8 recalled cosmetic products due to violations. Photo: Drug Administration of Vietnam |
List of the 8 recalled cosmetic products due to violations. Photo: Drug Administration of Vietnam
Health experts warn that products with incorrect formulas may contain unsafe ingredients, increasing the risk of adverse reactions and reducing their effectiveness. Substandard products can cause irritation, allergies, long-term skin damage, and potential toxicity from the absorption of harmful substances through the skin.
Yesterday, the DAV also announced the recall of eight Image brand cosmetics distributed by Minh Khuong Trading Company Limited (TPHCM). The recall was due to discrepancies between the ingredients listed on the product labels and the registered formulas.
In 2024, the DAV recalled 500 product registration numbers for imported cosmetics at the request of companies voluntarily withdrawing their products, and over 100 numbers due to regulatory violations.
Le Nga