On 24/9, two Indian pharmaceutical companies, Dr Reddy's Laboratories and Hetero Labs, announced plans to produce and distribute a generic version of Lenacapavir starting in 2027. This price is expected to apply to 120 low- and middle-income countries, which bear the greatest burden of HIV.
Developed by Gilead Sciences (US) under the brand name Yeztugo, Lenacapavir is a breakthrough in HIV prevention. Administered as an injection twice a year (every 6 months), it has demonstrated near-perfect effectiveness in preventing infection during large-scale clinical trials.
According to the World Health Organization (WHO), the HIV pandemic has claimed the lives of 44 million people and continues to infect 1.3 million annually. Experts, including doctors and activists, believe this injection could be a game-changer in the fight against HIV/AIDS.
At 40 USD per year, the generic version is significantly more affordable than the 28,000 USD annual cost of the brand-name Yeztugo in the US. This will provide millions in developing nations with access to advanced prevention methods.
"Producing a generic Lenacapavir is crucial to ensure this groundbreaking method isn't limited to the privileged few," emphasized Professor Saiqa Mullick, Scientific Director at the Wits Reproductive Health and HIV Institute (South Africa).
This production agreement is the result of international collaboration. Unitaid, a global health agency sponsored by WHO, provides technical and financial support to Dr Reddy's, along with the Clinton Health Access Initiative. The Gates Foundation is partnering with Hetero.
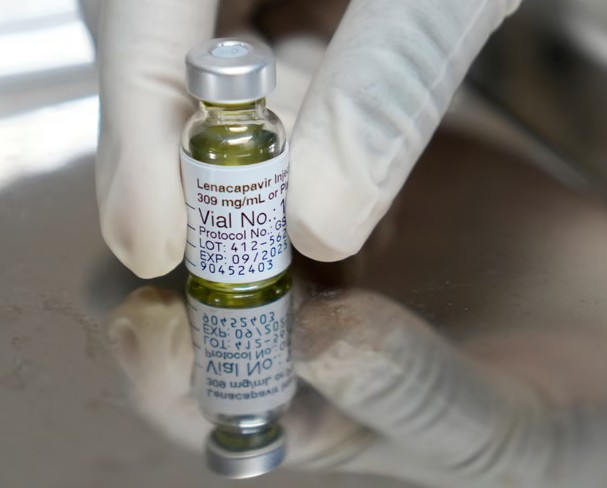 |
Lenacapavir launched in the US with a list price of over 28,000 USD per year. Photo: AP |
Lenacapavir launched in the US with a list price of over 28,000 USD per year. Photo: AP
Carmen Perez Casas, Head of HIV Strategy at Unitaid, stated that the 40 USD price is comparable to the current cost of oral PrEP medication. However, the 6-monthly injection will address barriers for those who struggle with daily adherence due to stigma or logistical challenges. This is part of a royalty-free licensing agreement Gilead signed with six manufacturers last year, allowing them to sell the drug in 120 countries upon approval.
However, Gilead's agreement has drawn criticism from advocacy groups for excluding upper-middle-income countries, particularly in Latin America. Casas said Unitaid is supporting these countries, like Brazil, which participated in trials but is excluded from the generic access list, in finding ways to overcome access barriers.
While awaiting the generic manufacturers, Gilead is working with the Global Fund to Fight AIDS, Tuberculosis and Malaria and the US government to provide the brand-name drug at a discounted price to two million people starting this year. However, experts estimate the actual need exceeds 10 million, highlighting the importance of widely available, affordable generics.
"The availability of affordable generics will dramatically multiply the impact of this landmark initiative," shared Peter Sands, Executive Director of the Global Fund.
Binh Minh (Reuters)