The regulatory agency identified Ucanrely Co., Ltd., based in Hanoi, as having violated cosmetic management regulations. The company failed to provide the mandatory Product Information File (PIF) during an inspection. This technical document is essential for businesses to retain, proving the quality, safety, and efficacy of their cosmetic products.
Regulations state that businesses are not required to submit a PIF when announcing product circulation. However, the entity responsible for bringing products to market must present the PIF immediately upon request by authorities. Without this file, the business will have its product registration numbers revoked and face penalties for the violation.
On 21/1, the Drug Administration of Vietnam (DAV) announced the recall of 77 cosmetic products. The recalled items include shampoos, conditioners, toners, facial cleansers, moisturizers, sunscreens, body lotions, hand creams, toothpastes, and various other facial care products. Popular brands such as Hatomugi, Ichimaki, Softymo, Rosette, Arau Baby, Fino, and Pantene are among those on the recall list.
The DAV has revoked the cosmetic product announcement numbers for all 77 items. It has also ordered the suspension of circulation, nationwide recall, and destruction of these products, with a report due by 25/1. Businesses that fail to comply with the recall or continue to sell these products will face legal action.
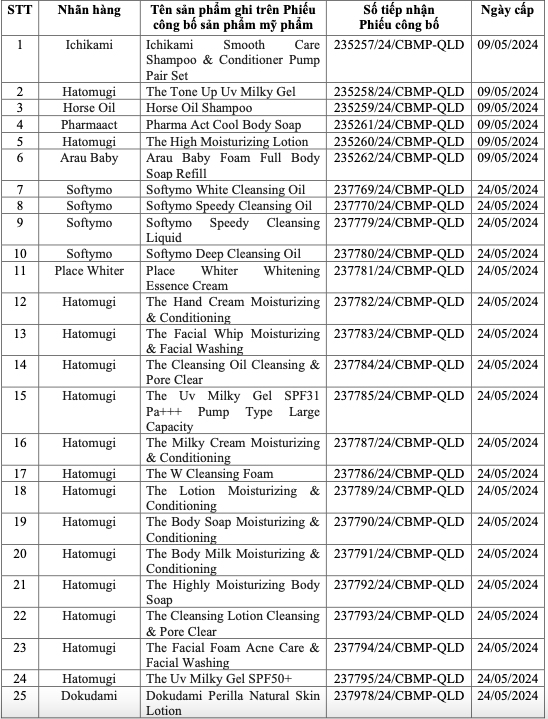 |
List of recalled products. Photo: Drug Administration of Vietnam
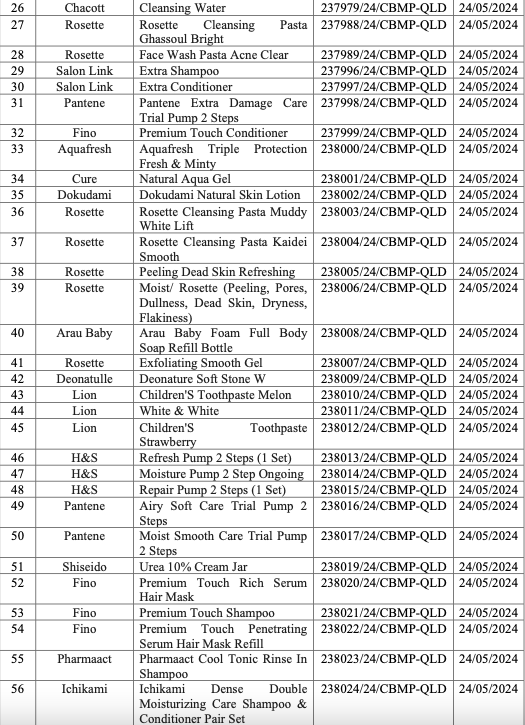 |
List of recalled products. Photo: Drug Administration of Vietnam
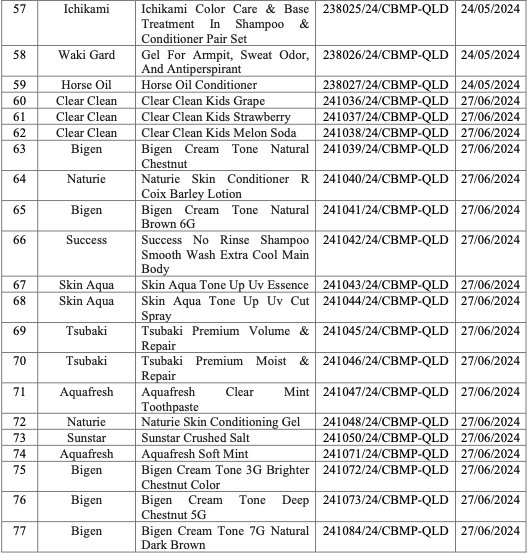 |
List of 77 recalled products. Photo: Drug Administration of Vietnam
Since early January, the DAV has recalled and destroyed numerous substandard cosmetic products. These products contained prohibited substances, undeclared ingredients, or lacked proper registration numbers. On 20/1, three types of popular hand washes and facial creams were recalled for containing sodium benzoate, an ingredient not listed in their product formulas. Last week, 8 cosmetic products were also recalled because they contained Miconazole, an antifungal agent no longer permitted for use in cosmetics.
As the Tet (Lunar New Year) holiday approaches, the demand for health and beauty products typically rises. In response, authorities are intensifying inspections of cosmetic businesses and market surveillance to ensure product quality and safety.
Le Nga