The regulatory body stated that this action complies with the ASEAN Cosmetic Agreement. Previously, at its 42nd session, the ASEAN Cosmetic Committee and the ASEAN Cosmetic Scientific Committee agreed to add Miconazole and Miconazole nitrate to the banned list. Experts have identified these as medicinal substances for treating illnesses, requiring a doctor's prescription and supervision. They are unsuitable for consumers to use independently and long-term in cosmetic forms like shampoo or feminine wash. The new regulation clearly distinguishes between drugs and cosmetics, preventing public health risks.
The list of 8 products mandated for destruction in this phase includes various anti-dandruff shampoos and feminine hygiene solutions. Among them, Duoc Hau Giang Joint Stock Company has three products: MicoFem Silk, MicoFem Bloom, and MicoFem Calm. TEDIS - Viet Ha Pharmaceutical Joint Stock Company is responsible for distributing two shampoos: Biorga Cystiphane Normalizing Anti-Dandruff Shampoo S and Biorga Cystiphane Intensive Anti-Dandruff Shampoo DS.
The remaining products include Piajour Woman Body Foam from HKK Trading Limited Company and two types of Dermavia Egeria Minazole Anti-Dandruff Shampoo, which belong to BIOPHAR Vietnam Trading and Import-Export Limited Company.
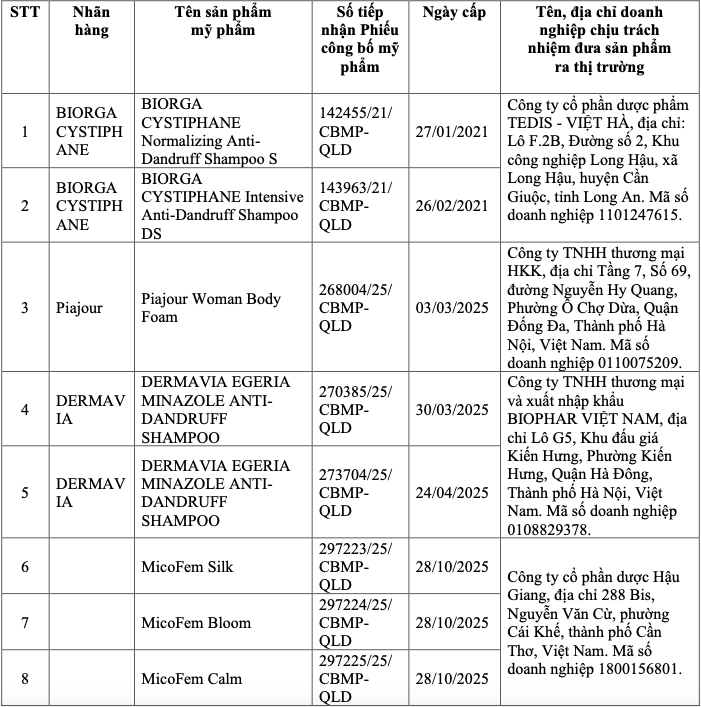 |
List of recalled products. Photo: Drug Administration of Vietnam.
The Drug Administration of Vietnam has instructed the involved businesses to issue urgent notifications to distribution systems, proceed with recalling, and destroy all non-compliant batches. Provincial and city health departments are responsible for supervising this process and imposing strict penalties on non-compliant entities. Authorities also advise the public to cease buying, selling, or using the aforementioned products and to return them to their suppliers.