These products include facial cleansers, masks, moisturizing creams, and skin repair treatments, under the Derma Peel and Derma+ brands. They are manufactured in Korea and distributed by Trinh My Co., Ltd. in TP HCM. The specific products are: Derma peel Rejuvenate, Derma peel -Max Brightening Stem Cell Solution, Derma peel Peptide Waterful Cream, Derma peel One - Touch, Derma peel 02 Bubble Foam Cleanser, and Derma+ Soothing Mask.
According to the Drug Administration of Vietnam, the products were recalled because their product information files (PIF) were incomplete and their actual formulas did not match the declared information. This constitutes a violation of cosmetic management regulations.
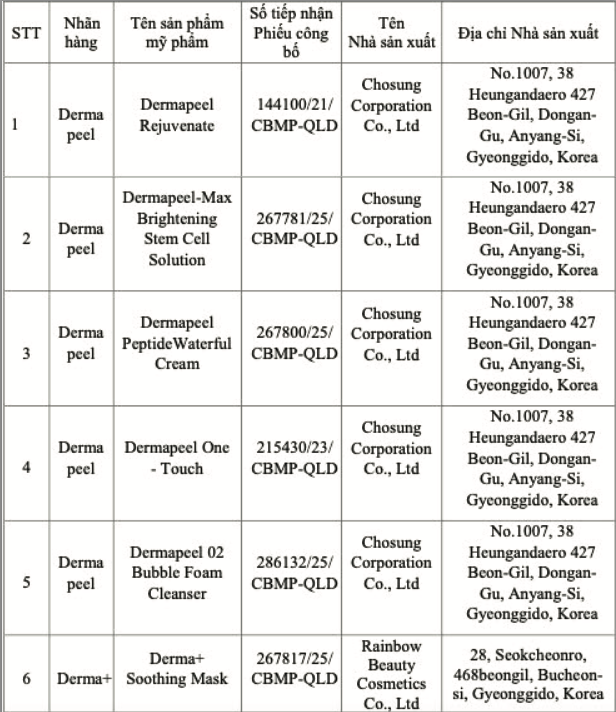 |
List of the six recalled products. *Photo: Drug Administration of Vietnam* |
Following this violation, the Drug Administration of Vietnam ordered an immediate halt to the sale and use of the six cosmetic products. Companies were instructed to return the products to their suppliers and report compliance by 5/3.
The Ministry of Health also revoked the six product announcement receipt numbers for the violating cosmetic products. Additionally, it suspended the review and acceptance of cosmetic product declaration dossiers from Trinh My Co., Ltd. for six months.
Since early January, the Drug Administration of Vietnam has recalled and destroyed numerous substandard cosmetic products. These products contained banned substances, undeclared ingredients, or lacked registration numbers. On 20/1, three types of hand sanitizers and facial cleansers widely sold were recalled for containing sodium benzoate, which was not listed in their formulas. Last week, eight cosmetic products were also recalled because they contained Miconazole, an antifungal active ingredient that has been removed from the list of permitted cosmetic ingredients.
These decisive actions by the Drug Administration of Vietnam aim to tighten market discipline and protect consumer health amidst a growing number of violations.
Le Nga