On 9/9, eGenesis Bio, the biotechnology company that developed the genetically modified pig kidneys, announced it had received FDA approval. The pig kidneys have been genetically engineered for compatibility with humans. Scientists used CRISPR-Cas9 technology to edit 68 pig genes, preventing rejection. They also removed three genes on the surface of pig cells that the human immune system might recognize and attack. At the same time, they deactivated retroviruses, which can infect humans. This breakthrough offers new hope for more than 100,000 American patients waiting for donor organs.
Paul Conway, chair of policy at the American Kidney Fund, called it "an incredibly optimistic period" amid a severe shortage of donor organs.
Before the large-scale trial, several transplants were performed with promising results. Most recently, Massachusetts General Hospital (MGH) transplanted a pig kidney into its third patient, 54-year-old Bill Stewart. After the surgery in June, he recovered and returned to work.
Stewart, an athletic trainer, has a history of high blood pressure and had been on dialysis three days a week for over two years due to declining kidney function. He has blood type O, which often means a 10-year wait for a human kidney.
Two previous MGH patients also received pig kidneys. The first, Rick Slayman, died after two months from a heart issue unrelated to the transplant. The second, Tim Andrews, remains healthy and is the world's longest-surviving pig kidney recipient. Other pig heart and kidney transplants have been performed at various medical centers with mixed results.
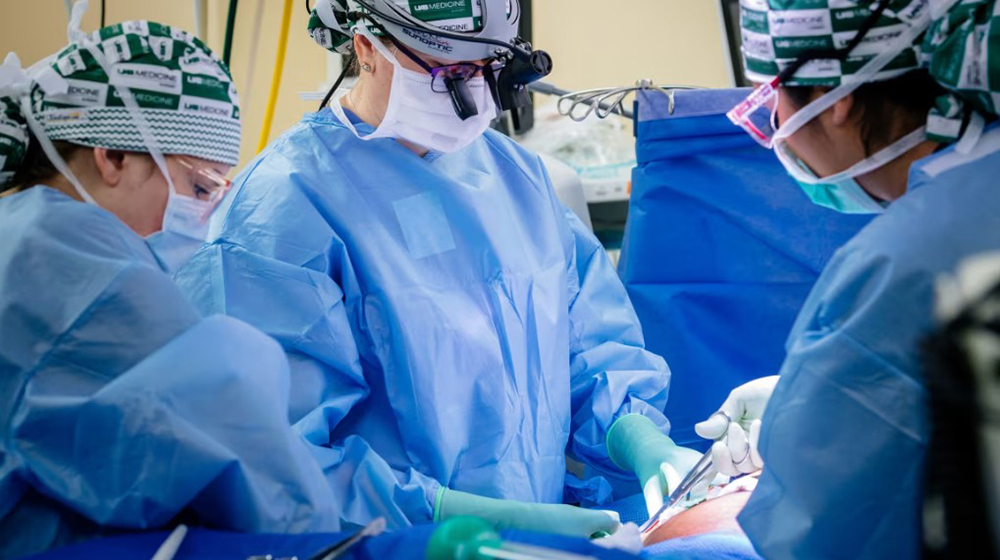 |
A research team from the University of Alabama at Birmingham biopsies a genetically modified pig kidney. Photo: Steve Wood/University of Alabama |
A research team from the University of Alabama at Birmingham biopsies a genetically modified pig kidney. Photo: Steve Wood/University of Alabama
The upcoming trial will help scientists evaluate the effectiveness and durability of the transplanted kidneys in a more diverse group of patients rather than individual cases. Mike Curtis, CEO of eGenesis, said the company plans to transplant 33 patients in the next two and a half years. Another company, United Therapeutics, also plans to start trials this year.
According to an American Kidney Fund survey, over 70% of respondents would accept a pig kidney transplant if approved by the FDA.
Dr. Leonardo Riella, a kidney transplant specialist at MGH, believes that testing healthier patients is key to verifying the durability of the transplants without the complications of long-term dialysis skewing the results.
Dr. Robert Montgomery, a pioneer in the field, expressed amazement at the speed of progress. He said "no one thought" there would be two FDA-approved trials in less than four years.
Binh Minh (CNN)