The vaccine has received 17.3 million USD in funding to support trials and ensure continued research and development. If proven effective and approved by regulatory agencies, the research team plans to create a vaccine stockpile for use in countries affected by the Nipah virus, enabling a rapid response to future outbreaks.
The vaccine is based on viral vector technology, the same technology used in a previously approved Ebola vaccine. This technology allows the vaccine to become effective quickly after vaccination. In the first phase of clinical trials, the vaccine demonstrated the ability to generate a rapid immune response after just one dose.
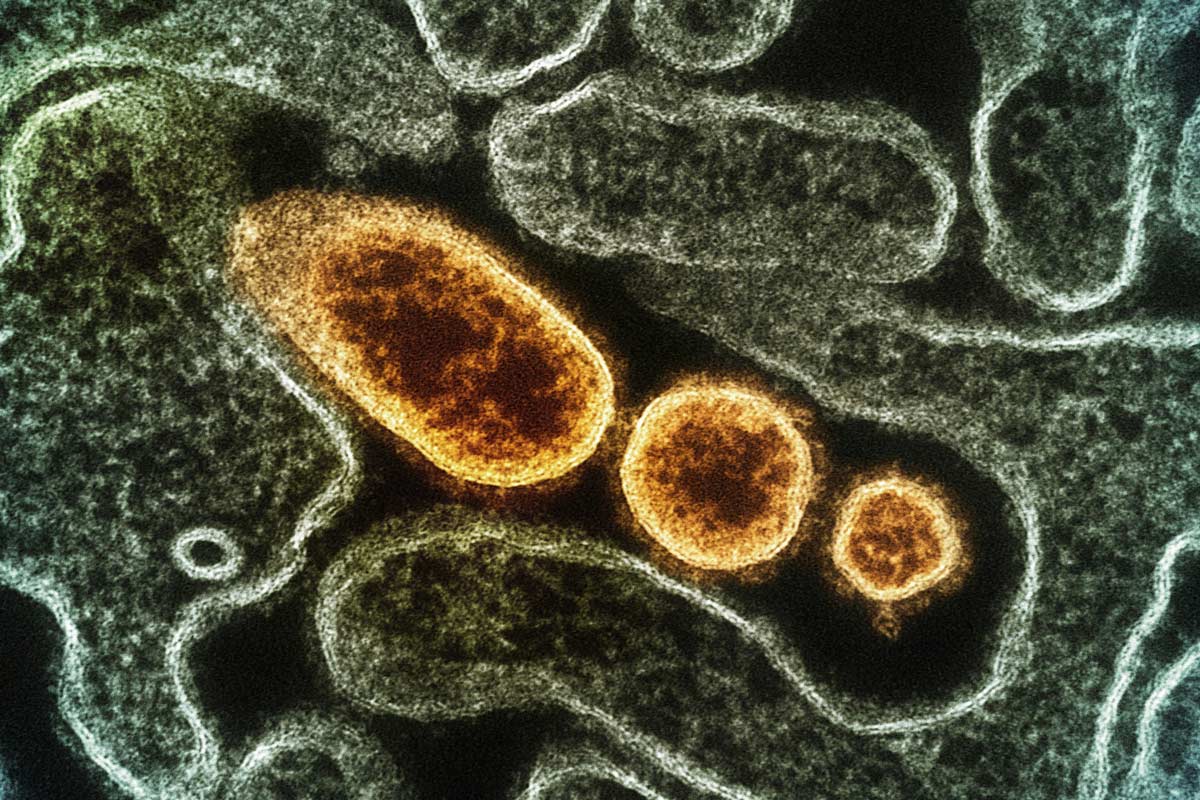 |
Nipah virus under a microscope, colored yellow. Photo: NIAID |
Nipah virus under a microscope, colored yellow. Photo: NIAID
Nipah is a virus belonging to the Paramyxovirus family and originates in animals. The pathogen is transmitted through food or directly from person to person. The virus causes a range of illnesses, from asymptomatic infections to acute respiratory illness and fatal encephalitis. Symptoms appear 4 to 14 days after infection. Patients with brain swelling can fall into a coma within 24 to 48 hours.
Currently, there is no vaccine or specific treatment for Nipah virus disease. According to the World Health Organization (WHO), the virus has a mortality rate of 40-75%. However, the 2018 outbreak in Kozhikode had a mortality rate of over 90%. Survivors often suffer long-term health problems.
The most recent cases have been reported in India and Bangladesh, likely spreading from bats and pigs to humans through close contact. In Asia, WHO has recorded several outbreaks in Malaysia and Singapore. Most human infections result from direct contact with infected pigs or contaminated tissue. Vietnam has not recorded any Nipah virus infections, but Ho Chi Minh City has increased surveillance for individuals returning from affected areas.
Chi Le (According to CEPI, WHO)