The recalled batch is Alfachim 4.2 tablets (Chymotrypsin (equivalent to 21 microkatal chymotrypsin) 4200 IU), batch number 03010624, produced by Cuu Long Pharmaceutical JSC. This anti-inflammatory drug is used to treat edema following injuries, burns, and surgeries.
In May, the National Institute for Drug Quality Control tested the drug and found it did not meet quality standards. The Drug Administration of Vietnam then ordered a recall of this batch in Hanoi. Cuu Long Pharmaceutical JSC was also instructed to collect additional samples and send them to either the National Institute for Drug Quality Control or the Ho Chi Minh City Institute for Drug Quality Control for quantitative analysis.
However, after the 15-day deadline, the company failed to report the results of the additional quality checks. They only reported on the drug's production, distribution, and the initial recall. Subsequently, they sent a document to the Drug Administration of Vietnam requesting a voluntary recall of the batch.
On 22/6, the Drug Administration of Vietnam ordered Cuu Long Pharmaceutical JSC and its distributors to immediately recall the entire substandard batch.
The Hanoi and Vinh Long Departments of Health will oversee the recall and disposal process, assessing its effectiveness and determining if any affected products remain in circulation, potentially posing health risks to consumers.
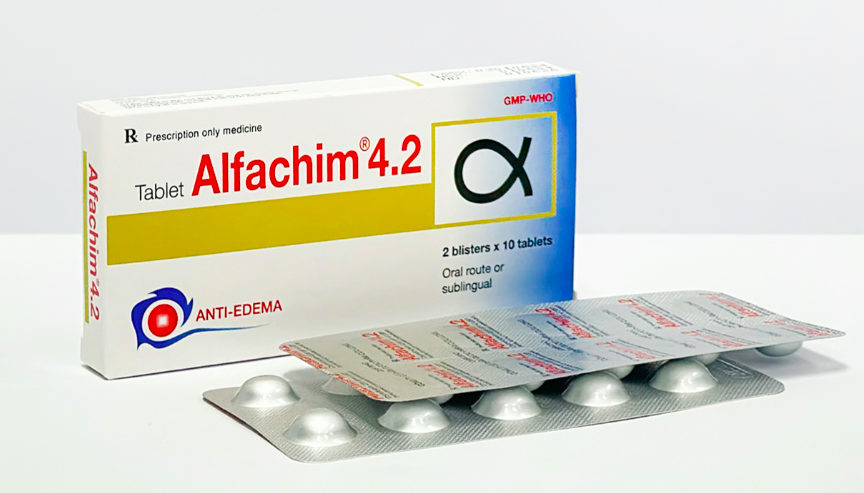 |
A batch of Alfachim 4.2 produced by Cuu Long Pharmaceutical JSC has been recalled nationwide. Photo: Cuu Long Pharmaceutical |
A batch of Alfachim 4.2 produced by Cuu Long Pharmaceutical JSC has been recalled nationwide. Photo: Cuu Long Pharmaceutical
Ta Manh Hung, Deputy Director of the Drug Administration of Vietnam, reported that following a month-long campaign against smuggling, commercial fraud, and counterfeit goods, authorities uncovered numerous violations at production and business facilities. They confiscated and destroyed many counterfeit and substandard drugs and cosmetics. Counterfeit and unidentifiable drugs commonly used for stomach ailments, flu, asthma, and diabetes were found in several pharmacies.
"Counterfeit and unidentifiable products in the health sector mainly involve functional foods, health supplements, traditional medicines, and cosmetics," Hung said. He added that in the past month, 17 out of 38 randomly inspected facilities were found to have committed violations. Twenty provinces and cities also formed inspection teams, checking 865 drug and cosmetic production, import, and trading facilities, uncovering violations at 48.
In response, the Ministry of Health is developing a comprehensive plan with solutions including: reviewing legal regulations to strengthen penalties and deter violations; enhancing inspection and supervision at all management levels; establishing a database of registered drugs, connecting pharmaceutical businesses to track the origin of drugs; and intensifying post-market surveillance, regular and surprise inspections.
Le Nga