Dr. Tran Hai Binh, Deputy Head of the Oncology Department at Tam Anh General Hospital Hanoi, explains that biologics, or biological products, are medicines derived from living organisms such as yeast, bacteria, animal cells, and even the human body. Blood is the most common biologic, collected from donors and then transfused to patients. Other biologics include: vaccines, insulin, stem cells, gene therapy, monoclonal antibodies, and immunosuppressants.
Biologics are categorized into immunotherapy and targeted therapy, currently used to treat various cancers. These drugs combat specific proteins on cancer cells, helping the immune system recognize and destroy them.
According to Doctor Binh, some biologics approved for cancer treatment since the late 1990s have seen their exclusive patent protection expire, leading to the development of numerous biosimilars.
 |
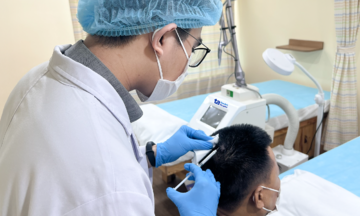
Doctor Binh advises cancer patients on using biologics. Photo: Tam Anh General Hospital |
Doctor Binh advises cancer patients on using biologics. Photo: Tam Anh General Hospital
A biosimilar is a near-identical copy of a reference biologic, manufactured from the same living organism source. It offers similar processes, quality, and benefits when used at the same concentration and dosage as the reference biologic, without causing new or more severe side effects.
Doctor Binh notes that biosimilars have minor differences compared to reference biologics. Biologics are inherently far more complex molecules than chemical-based drugs, making exact replication difficult. Furthermore, because biologics are produced in living cells, variations between product batches always exist, even when manufactured at the same facility by the same pharmaceutical company.
Biologics are currently considered an advanced cancer treatment, but their cost is relatively high. Pharmaceutical companies research, produce, and market biosimilars to compete with reference biologics. This competition, especially when multiple biosimilars exist for one reference drug, drives down drug prices.
Consequently, Doctor Binh believes that biosimilars offer lower prices with comparable efficacy to the original drugs, providing cancer patients with more treatment options.
In late October, the Ministry of Health approved the circulation of Russia's Pembroria in Vietnam for cancer treatment. This biosimilar contains the active ingredient pembrolizumab, similar to the US drug Keytruda, and is priced at approximately 18 million dong per vial, which is less than 1/3 the cost of the original drug.
Previously, the Ministry of Health had approved several biosimilars, including Herzuma, Trazimera, and Hertraz (containing the active ingredient trastuzumab, similar to the reference drug Herceptin) for breast cancer treatment. Additionally, other biosimilars for conditions such as non-Hodgkin lymphoma, leukemia, metastatic colorectal cancer, and non-small cell lung cancer have also received approval.
Thanh Long
| Readers can ask questions about cancer here for doctors to answer. |