Researchers from Rutgers University in New Jersey announced the successful creation of self-destructing plastic with a programmable degradation rate. Associate Professor Yuwei Gu from the Department of Biochemistry and his colleagues published this research last week in the scientific journal Nature.
Gu conceived this idea while walking through Bear Mountain Park in New York, observing plastic bottles scattered on paths and lakes to a "horrifying" extent. While both are formed from polymer chains, plastic persists for centuries, whereas natural proteins and DNA do not accumulate as waste. Gu stated that they drew inspiration from natural structures, aiming for plastic to disappear after its useful life.
The "timed" mechanism relies on the strategic placement of chemicals that break down plastic bonds. Researchers restructured the plastic to expose weak bond groups. Auxiliary chemicals are embedded near these bonds, awaiting an activating agent to rotate them into position for breakage, initiating the plastic degradation reaction.
 |
A sample of poly(dicyclopentadiene) plastic, a material used in car components, restructured to self-degrade within a few days at room temperature. *Photo: Gu Lab*
"We found that changing the chemical's position significantly impacts the degradation rate", Gu said. This means that by controlling the placement of these chemicals, chemists can create plastic with varying degradation rates: daily, monthly, or over many years. The closer the chemical is to the weak bond, the faster the product degrades.
This fine-tuning ability allows plastic products to have a lifespan suitable for their intended use. For example, plastic bags only need to last for one day before degrading, while car components require durability for several years. The research team demonstrated that degradation capabilities can be integrated into materials using additives, or actively triggered via ultraviolet rays and metal ions for enhanced control.
Gu explained the plastic restructuring process as similar to folding a piece of paper, making it easier and quicker to cut. This "pre-folding" of the structure during plastic manufacturing causes the material to degrade thousands of times faster when activated. However, under normal conditions, it remains durable and useful because its chemical composition is preserved.
Initial laboratory tests showed that the liquid produced during degradation is non-toxic. The scientists stated that further research is needed to ensure these byproducts do not harm ecosystems, ensuring the material's safety throughout its lifecycle.
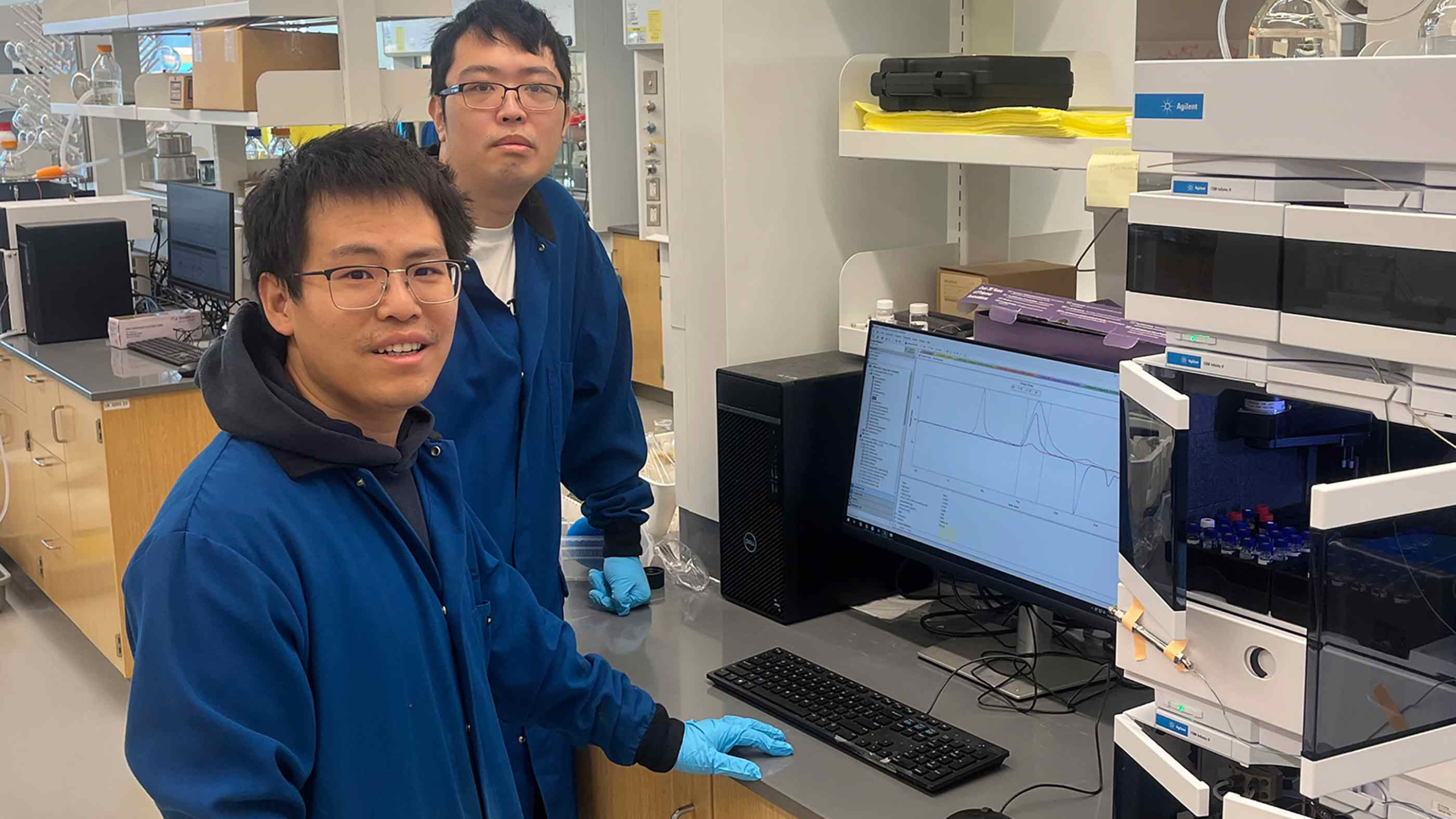 |
Chemist Yuwei Gu (left) and research student Shaozheng Yin (right) use a machine to measure polymer size and their degradation. *Photo: Gu Lab*
A polymer is a long chain of repeating basic components, similar to beads on a string. In nature, the basic components of DNA and RNA polymers are organic nucleotide compounds, and in proteins, they are amino acids. During degradation, in addition to bacterial assistance, natural polymer chains inherently integrate small helper groups that break chemical bonds at appropriate times.
The research by Gu and his colleagues introduces an environmentally friendly plastic and opens up a toolkit for designing smart polymer-based materials that meet degradation needs in various fields.
To date, this new material has only been created and tested under laboratory conditions. The research team needs to conduct more tests to ensure its safe use before introducing the new material to the market.
The research team is also exploring how to integrate their process with conventional plastics and existing manufacturing methods. Concurrently, based on their plastic research, they are experimentally producing drug release capsules with programmed timing.
Bao Bao (according to Rutgers, Interesting Engineering)












