Respiratory syncytial virus (RSV) is a leading cause of pneumonia, bronchiolitis, and severe respiratory failure in young children. There is currently no specific treatment for the disease; management primarily involves monitoring and respiratory support. More than 80% of children hospitalized with RSV were previously healthy and full-term. Many recent cases have required intensive care due to complications.
To prevent RSV in young children, Vietnam has approved the circulation of the Nirsevimab monoclonal antibody, which is being deployed in hospitals and the VNVC Vaccination System. Doctor Huynh Tran An Khuong, Medical Specialist at the VNVC Vaccination System, addresses five frequently asked questions about this antibody:
- What is the difference between monoclonal antibodies and vaccines?
Vaccines contain inactivated or weakened pathogens, prompting the body to produce antibodies after 2-3 weeks. However, infants, premature babies, and low-birth-weight infants have immature immune systems and may not mount a sufficiently strong response to RSV. Therefore, scientists developed the Nirsevimab monoclonal antibody by selecting antibodies from individuals previously infected with RSV, then multiplying and purifying them in laboratories to achieve high purity and safety for children. This complex process and high cost often make the price higher than vaccines.
- How do monoclonal antibodies work?
When administered to children, the Nirsevimab monoclonal antibody is immediately present in the blood. It can recognize and prevent the virus from entering cells and causing illness in the body. This provides immediate protection for children, independent of their immune system's maturity. For a disease that can rapidly progress to pneumonia and bronchiolitis, early protective efficacy is crucial in reducing the risk of severe illness and hospitalization for children.
 |
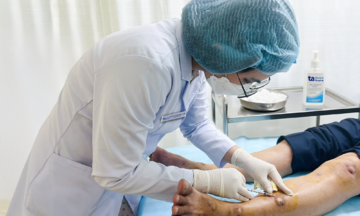
Children receiving RSV monoclonal antibodies at the VNVC Vaccination System. Photo: Tuan Anh
- Who can receive monoclonal antibodies?
Nirsevimab can be administered to children from one day old up to 24 months of age. It is suitable for all infants, including those who are premature, low-birth-weight, or have underlying medical conditions, with a safe profile and few reported post-injection reactions. Children from one day old to 12 months old receive one dose. Children over 12 months up to 24 months old who are at high risk of severe RSV disease receive two doses at two different sites on the same day. Dosage depends on the child's weight and the timing of administration.
- Do children who have had RSV need to be vaccinated?
Numerous studies indicate that children can be re-infected with RSV multiple times shortly after recovery, as the body does not develop lasting immunity. After two months, approximately 50% of children have been re-infected. After eight months, the re-infection rate increases to 67%. Within 26 months, up to 73% of children had been infected two or more times, and 47% had been infected three or more times. Therefore, children who have previously had RSV are still recommended to receive Nirsevimab monoclonal antibodies to reduce the risk of severe re-infection.
- What is the efficacy of monoclonal antibodies?
According to research data, Nirsevimab is 82,7% effective in preventing hospitalizations due to lower respiratory tract infections such as pneumonia and bronchiolitis caused by RSV. It is 75,3% effective in preventing very severe lower respiratory tract infections requiring hospitalization due to RSV. The protective effect lasts 5-6 months, corresponding to a typical RSV season.
Additionally, data also shows that Nirsevimab helps reduce hospitalizations for general lower respiratory tract infections from all causes in children.
 |
Young children, especially those under six months old, are at high risk of illness and severe complications when infected with RSV. Photo: Vecteezy
- Is a 5-6 month protective efficacy too short?
The World Health Organization (WHO) estimates that half of the more than 100,000 annual RSV deaths occur in children under six months old; in the US, 75% of hospitalizations happen during this period. In tropical countries like Vietnam, RSV circulates year-round, and there is no specific treatment. Nirsevimab monoclonal antibodies provide early protection for children with just one dose, lasting 5-6 months, reducing the risk of complications, death, and the burden on the healthcare system.
Therefore, this duration is not short. Furthermore, Nirsevimab utilizes special technology to extend the antibody's presence in the body, eliminating the need for multiple booster injections like older generation antibodies. This means that with a single dose, children are protected throughout the entire RSV season.
Gia Nghi